Does Glyoxal Contribute Significantly to Regional SOA Formation?
Submitter:
Knote, Christoph — Atmospheric Chemistry Division
Hodzic, Alma — National Center for Atmospheric Research (NCAR)
Area of research:
Aerosol Processes
Journal Reference:
Science
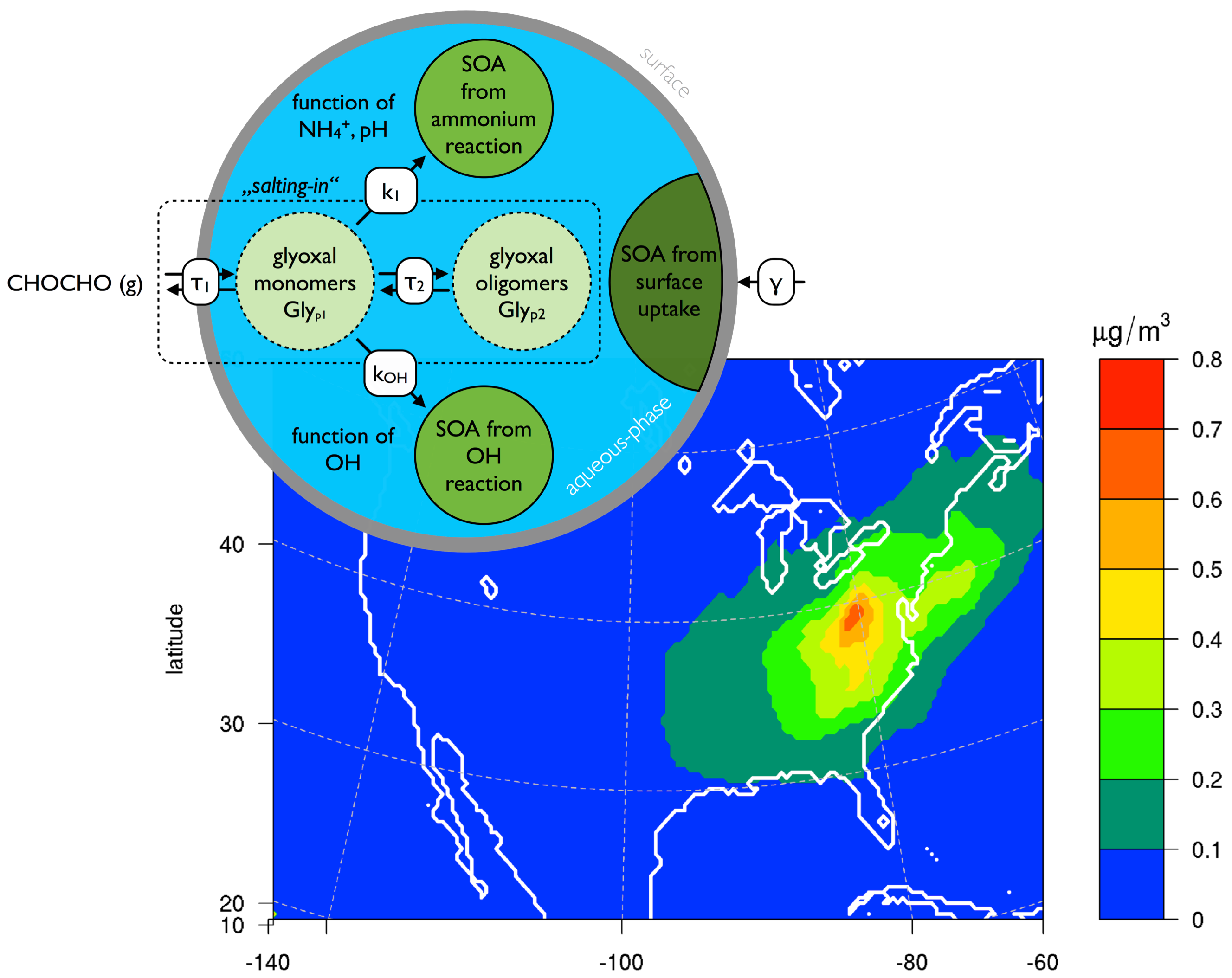
Organic matter is a major contributor to total ambient aerosol load. Therefore, understanding its formation and removal is key to mitigate air pollution problems and climate change. Recent research suggests that apart from the traditional formation processes through the gas-phase oxidation of gaseous precursors, in-cloud and in-particle chemistry could contribute substantially to ambient organic aerosol mass. Glyoxal is one of the precursor substances proposed to be important, and its aerosol formation potential has been shown in lab experiments as well as box and global modeling studies. However, a regional scale study, in which we could still consider a detailed representation of the chemical and physical properties of the aerosol while allowing for a realistic description of the variability in meteorology and biogenic as well as man-made pollution levels, has been missing.
Impact
In our study (Knote et al. 2013), we included the state of knowledge on secondary organic aerosol (SOA) formation from glyoxal into the regional chemistry transport model WRF-chem and conducted simulations (1) over California for the time period of the CARES/CalNex field campaigns, and (2) over the continental United States during summer 2010. We made use of the extensive measurement data set collected during the CARES/CalNex campaigns to constrain sources and concentrations of glyoxal, and its major precursors. We found that over California the South California Air Basin (SCAB) is the hotspot for SOA formation from glyoxal, due to the high availability of precursors, aerosols, and humidity. The eastern slopes of the Central Valley with their high isoprene loadings did not show substantially elevated levels of glyoxal SOA, despite the fact that isoprene is a major precursor for glyoxal. This is because of rather dry conditions that introduce kinetic limitations in the rate of glyoxal-SOA formation (Kampf et al. 2013). The exact formation mechanisms of SOA from glyoxal are still under debate. We have conducted a series of simulations to explore sensitivities and assess the potential atmospheric relevance of current hypothesis, i.e., surface vs. volume chemistry. We found that SOA formation chemistry in the bulk liquid phase of aerosols leads on average to a 1 percent contribution to total SOA mass, while a simple parameterization under the assumption of a surface process suggests that up to 15 percent of total SOA mass is actually from glyoxal in the SCAB. Reversible partitioning of glyoxal into the aerosols (e.g., through oligomerization) has been found to be negligible in its mass contribution. The reason for the lower SOA mass yields when looking at volume chemistry does not seem to be caused by slow in-particle chemistry, but rather limited by the efficiency to transfer glyoxal from the gas-phase into the aerosols.
Summary
Our study showed that novel pathways to form SOA could contribute considerably to total ambient aerosols load and, hence, cannot be neglected. However, our understanding of these processes is still quite limited and further study is necessary.